About pH : pH Measurement Method
When an electrode made of a special glass film that responds to hydrogen ions is immersed in the liquid to be measured, electromotive force corresponding to hydrogen ion activity is generated on the surface of the glass film. Since the generated electromotive force cannot be obtained by itself in theory, it is displayed on the voltmeter (pH meter) as the potential difference with the reference electrode when a voltmeter is connected between the device and the standard electrode. The index representing hydrogen ion activity is called "pH", and the relationship between pH and hydrogen ion activity [H+] is pH=-log[H+].
When measuring pH, if the pH meter is calibrated with a pH standard solution whose pH value is known beforehand, the pH value can be directly known from the electromotive force generated corresponding to the pH.
Here, an electrode having a special glass film responsive to pH is called glass electrode and standard electrode is called reference electrode. Reference electrode is provided with a high concentration liquid of potassium chloride inside and a liquid junction at its tip.
The liquid junction is a part in contact with the liquid to be measured, and porous ceramic is generally used. Reference electrode is in contact with the liquid under test at the liquid junction, but constantly generates a constant electromotive force and uses this electromotive force as a reference to determine the potential difference from the glass electrode.
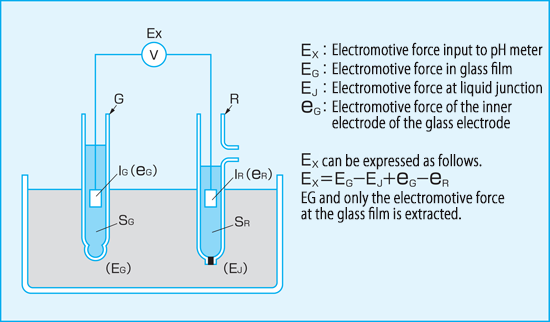
The theoretical formula for pH measurement can be theoretically expressed as the following formula.
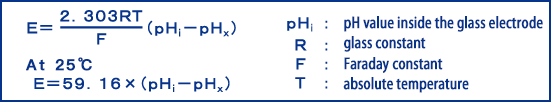
Here, 59.16 (25°C) is the theoretical slope, which is the potential difference when it changes by 1pH. This slope is a function of temperature, as can be seen from the above equation, and therefore changes with temperature. The pH meter is designed to compensate for the temperaturee-dependent slope using a temperature compensation method so that the pH can be measured correctly.
